LUDOVIC MARTINET
Finding novel immunotherapeutic strategies
against myeloma.
Promising results obtained by monoclonal antibodies in cancer suggest that immunotherapy will represent the future of myeloma treatments. We recently started to develop within our team a research axis dedicated to the development, testing and understanding of novel immune based therapeutic approaches against myeloma.
Improving anti-CD38 antibodies through Fc-engineering.
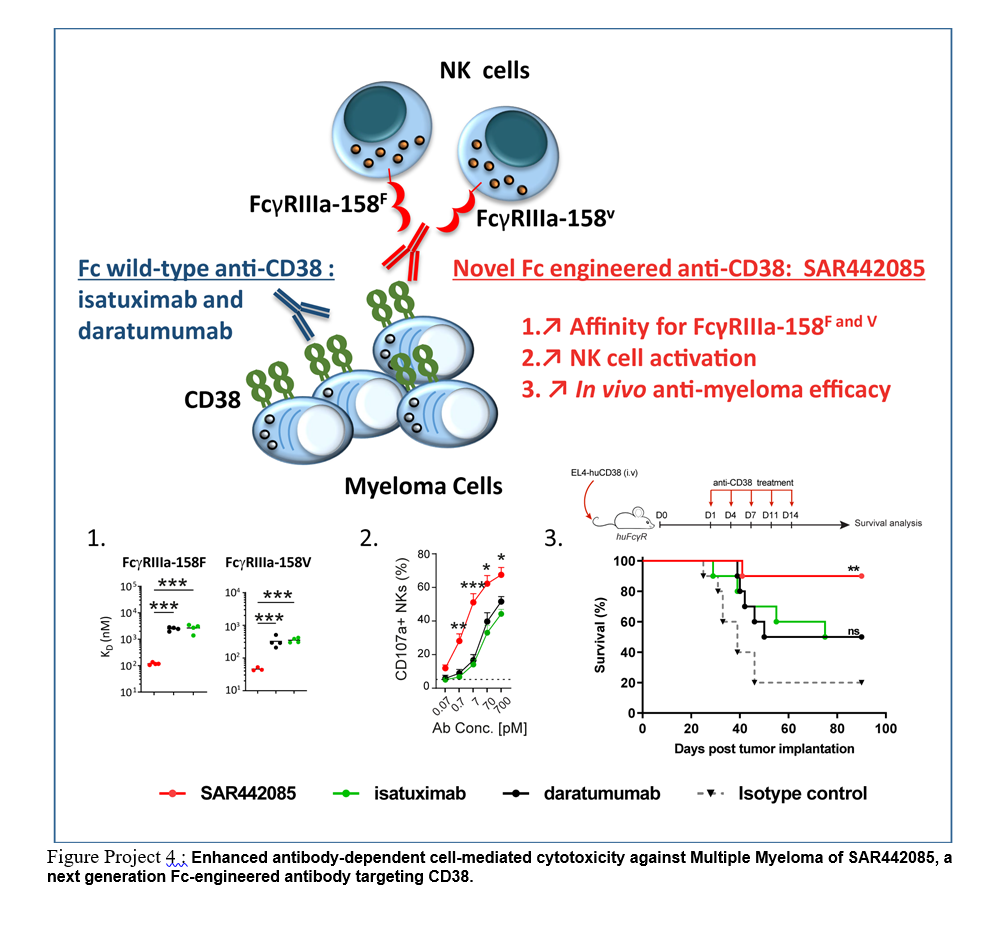
Daratumumab (Janssen) and Isatuximab (Sanofi), are mAbs targeting CD38 over-expressed by malignant plasma cells recently approved for the treatment of myeloma. Despite the clinical success of these agents, incomplete responses, resistance and relapses are common. The importance of immune dependent mechanisms in the efficacy of anti-CD38 mAbs, suggests that increasing their affinity towards activating FcγR may represent a promising therapeutic strategy to improve their clinical efficacy. In collaboration with Sanofi, we recently showed the preclinical efficacy of SAR442085, a next generation antibody derived from Isatuximab bearing ADE mutations, with higher affinity for the activating FcγRIIIa and FcγRIIa receptors (Kassem et al, Blood 2018). Fc mutated SAR442085 thathad higher anti-tumor efficacy than parental Isatuximab and Daratumumab is actually tested in phase I clinical trial (https://clinicaltrials.gov/ct2/show/NCT04000282) at iuct-oncopole.
Redirect T cells against myeloma targeting T cell bispecific engager
Bispecific T cell engagers (TCB) are valuable tools to improve the clinical efficacy and safety of mAb based immunotherapy. TCB are designed to simultaneously target tumor specific markers and T cell activating receptor thus redirecting T cell cytotoxicity against cancer cells. The majority of TCB targets CD3 and promotes TCR activation of T-cell at the proximity of tumor cells. This therapeutic strategy has considerably been developed over the past 10 years. Bispecific antibodies including JNJ-64007957 (Teclistamab, Janssen) and JNJ-64407564 (Talquetamab, Janssen) showed important preclinical efficacy and therapeutic promise against myeloma. Yet, many open questions related to their mechanism of action and determinants of efficacy/resistance remain to be addressed. Filling these gaps is a prerequisite to improve the efficacy of these agents. To address this question, we are actually performing immune profiling of myeloma patients along treatment with bispecific antibodies. In addition, in collaboration with pharma companies (SANOFI, JANSEN, ROCHE), we are actually testing the in vitro efficacy and specificity of novel T cell bispecific Abs against myeloma specific markers such as GPRC5D and CD38.
Finding novel immunotherapeutic strategies against myeloma.
Part of our project are dedicated to the preclinical testing of targets identified in our other projects alone or in association with existing therapies using cancer patients’ samples and relevant tumor mouse models.