
LUDOVIC MARTINET
Cytotoxic cell landscape controlling myeloma development and clinical outcome
Despite recent progress in therapeutic options, myeloma patients often experience multiple relapses even after successful therapy. Recent studies highlight the influence of the immune microenvironment in the pathogenesis of this disease. Both Natural killer (NK) and CD8+ T lymphocytes can recognize and destroy malignant myeloma cells. This point was substantiated by our Team using VK*MYC transgenic mice, the most relevant mouse model of myeloma. In this model, NK and CD8+ T cells not only limit spontaneous myeloma development in vivo, but they also impacted the therapeutic efficacy of standard chemotherapy used in the management of myeloma (Guillerey, Ferrari de Andrade et al. 2015). Thus understanding the immune landscape associated with myeloma development and the critical immune parameters associated with myeloma clinical outcome may allow the development of novel immunotherapies to limit the emergence of resistant myeloma clones.
Immune landscape associated with Multiple myeloma development and outcome.
The key NK and CD8+ T cell changes associated with myeloma development and outcome remain unknown. To address this question, we are currently analyzing at the single cell level transcriptomic landscape of NK and CD8+ T cell infiltrating myeloma lesions using CITE-seq technology. These results obtained on a restricted number of myeloma patients are then generalized and extended by flow cytometry analysis and in vitro functional assays on samples routinely collected in the laboratory at different stages of myeloma including premalignant MGUS, asymptomatic SMM, diagnosis and relapse. The clinical impact of key NK and CD8+ T cells populations and markers are then addressed by flow cytometry on biobanks of frozen bone marrow aspirates collected at diagnosis and upon relapse.
Metabolic changes controlling Multiple myeloma immune surveillance
It is now clear that metabolic deregulations may be at the origin of tumor infiltrating lymphocyte dysfunction. Indeed, the increased utilization of glycolysis and oxidative phosphorylation (OxPHOS) by tumor cells often leads to a depletion of essential nutrients, as well as the accumulation of immunosuppressive metabolites. Hence, the functionality of immune cells may not only be affected by their intrinsic metabolic alterations, but also by the unfavorable metabolic milieu within the tumor microenvironment. This may be the case in myeloma as we recently observed metabolic alterations in NK cells from myeloma patients as compared to HD. Yet, until now, very little information is available around the immune metabolic alteration in the bone marrow of myeloma patients.
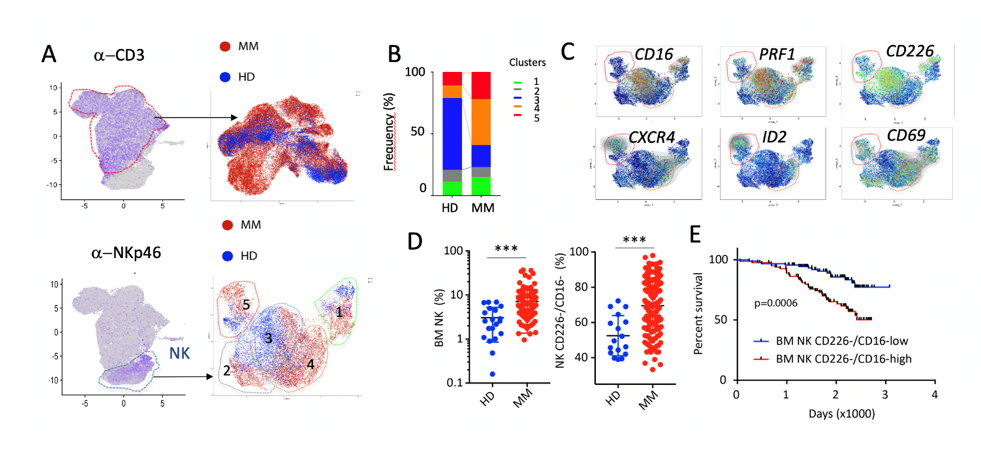
Figure 1: Stratification and clinical outcome of MM patients according to cytotoxic cell landscape. (A). Identification of NK and CD3+ T cells on UMAPs calculated with CITE-seq data of paired Blood and BM samples from 4 HD and 4 MM patients at diagnosis. (B-C) T cell (B) and NK cell (C) clusters in the BM of MM and HD. (D) Relative frequency of MM and HD NK cells clusters. (E) Differentially expressed NK cell selected genes between MM and HD. (F) Relative frequency of BM NK cells and CD226-/CD16- NK cells in the IFM2009 (n=180) cohort compared to HD (n=20). (G). Survival estimates for MM patients with high (>median, red) or low (<median, blue) infiltration of CD226-/CD16- NK cells. Log-rank (Mantel-Cox) test.

