
Impact of tumour immune microenvironments on the response to immunotherapies
Christel Devaud
Our work focuses on the pro- or anti-tumour polarisation of the immune microenvironment in epithelial tumours. We have developed several preclinical models, all based on orthotopic implantation of different cancers (i.e. directly into the tumour site of origin) and in-depth monitoring of the progression, using an in vivo bioluminescence camera. Previously, these models have highlighted two novel concepts: 1/ the polarisation of the tumour immune microenvironment is essentially based on tumour localisation and 2/ primary tumours can impact distant secondary tumours and modulate their microenvironment.
Our current projects explore the complexity of epithelial tumour microenvironments and aim to develop new immunotherapies. We have recently published a study on colorectal cancer, focusing on the bipolarity of the immune microenvironment of primary colonic tumours, influencing tumour progression or rejection. The remainder of this project focuses on the distant anti-tumour impact of colonic tumours on liver metastases, which are particularly difficult to treat. We use innovative multi-tumour models localised in the primary (colon) and metastatic (liver) sites of the animals. This project aims to determine, in patients, cellular and molecular signatures characterising metastatic colorectal cancers that are potentially responsive to immunotherapies. Another ongoing project concerns cancers of the ENT sphere (upper aerodigestive tract) with other orthotopic models (ENT implantation in animals) and aims to understand the mechanisms of resistance to immunotherapies in these cancers. The data we obtain through preclinical tools are systematically extended to a translational dimension using patient cohorts constituted through strong interactions with the Toulouse Oncopole University Cancer Institute.
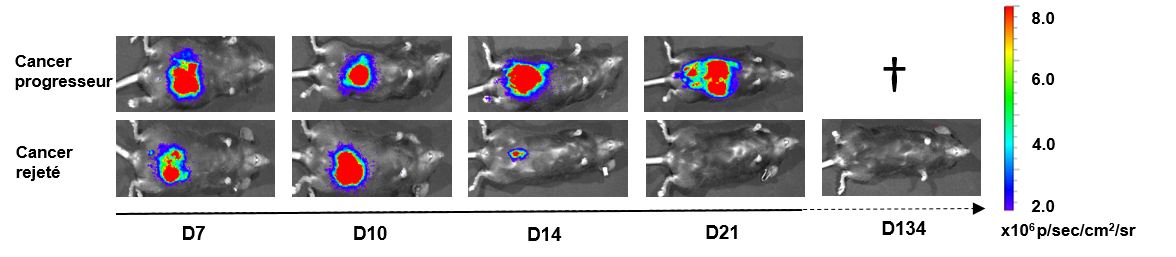
Suivi de souris C57Black/6 avec des tumeurs coliques bioluminescentes et un cancer progresseur ou rejeté / Monitoring of C57Black/6 mice with bioluminescent colonic tumours and progressive or rejected cancer
Technologies used:
Microsurgery for preclinical tumour implantation (mouse models: intra-colon, intra-hepatic, intra-jowl, intra-vagina, intra-kidney, intra-lung, intra-peritoneum…); In vivo imaging monitoring (2D and 3D; IVIS® camera, PerkinElmer); RNASeq; single-cell RNAseq; Nanostring technology; Bioinformatics analysis; Flow Cytometry (17-colour labeling).

