
Pharmacokinetic modelling of radiolabelled antibodies to understand resistance mechanisms in immunotherapy.
Dr Mélanie White-Koning
Using Position Emission Tomography (PET) imaging, we are studying the evolution of PDL1 (Programmed Death Ligand-1) markers during immunotherapy treatment by labelling an anti-PDL1 antibody with zirconium 89 (89Zr), a radioisotope whose physical peroxide is compatible with the pharmacokinetics of monoclonal antibodies. The study of in vivo pharmacokinetics in syngeneic mouse models of non-small cell lung cancer expressing PDL1 will make it possible to determine the distribution, metabolisation and elimination of radiotracers after intravenous injection, as well as their tumour distribution (sensitivity, specificity). Blood and organ samples were taken to model radiotracer distribution and validate its application in vivo. In these mouse models, with and without treatment, the survival of the animals and the evolution of the tumour and its microenvironment using histological sections were also studied. Analyses are still in progress concerning the use of population pharmacokinetic methods to study the distribution of the tracer and estimate the best imaging time.
The treatments used in this study are immune checkpoint inhibitors such as nivolumab or pembrolizumab, depending on the current administration schedules in humans. Once validated, these radiotracers could be used to visualise changes in markers over the course of the disease and to characterise patients who may respond to immune checkpoint inhibitors. This will provide a better understanding of the mechanisms of resistance to these treatments and the role of the tumour microenvironment in their acquisition.
Financing :
- CIFRE (Zionexa-GE)
- FEDER (Europe –Région)
Collaboration
- TONIC (Inserm UMR 1214),
- ex-Zionexa (now GE),
- projet PiR²
Illustration :
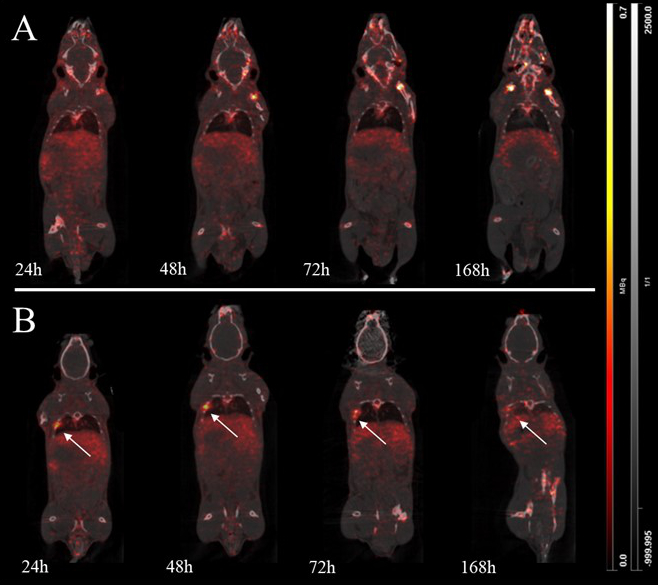
Imagerie préclinique de [89Zr]DFO-anti-PDL1 dans des souris saines (A) et des souris avec tumeur pulmonaire (B) / Preclinical imaging of [89Zr]DFO-anti-PDL1 in healthy (A) and lung cancer grafted mice (B).
partnership



